Defect of the Month
Back to AGR's Library
This v-shaped notch is a glass forming flaw referred to as a “Notch Defect”. While not easily visible, the notch defect will greatly reduce glass strength in pharmaceutical vials and must be avoided. The picture shows a circumferentially aligned fracture located on the underside of the flange near the barrel junction. This type of breakage results from an upward directed force applied to the underside outer edge of the flange, generating tensile stress near the base of the flange/barrel junction. Due difficulties in viewing the notch defect, some glass manufacturing companies have started to view this junction under magnification in an optical comparator.

Lost in the murky depths of internet lore, some unknown wag coined the terms “nope rope” and “danger noodle” as quirky alternate names for snakes. As is the way with the internet, these catchy names quickly became memes that linger in cyberspace. The term “nope rope” could also be applied to cord in glass containers. In fact, the Spanish word for cord is ‘cuerda,’ which literally means ‘rope’. Cord is a streak of glass that has a somewhat different composition from the surrounding glass, altering its physical and optical properties. When a cord streak causes high tensile stresses, it can lead to reduced performance, primarily in the form of delayed breakage after filling. Proper cord measurement techniques are therefore essential to detected and rejecting excessive cord. So when it comes to highly stressed cord, just say ‘nope!’
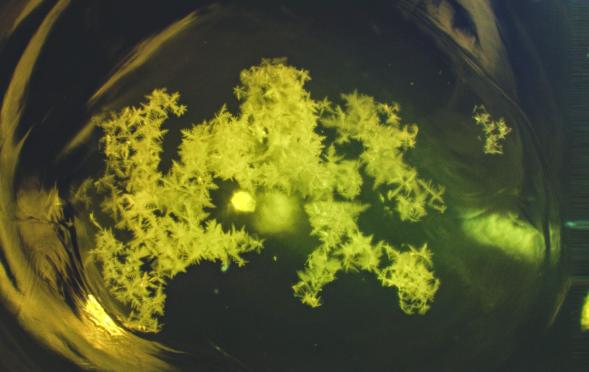
Here at AGR, we never get tired of seeing zirconia dendrite stones in glass containers. The little snowflake crystals swarm in the middle of a glassy knot enriched in alumina, a bit like dandelion tufts floating in a puddle or a star cluster observed through a telescope. These stones are created when AZS refractories in the melter erode and drip down into the molten glass. Upon cooling, the zirconia recrystallizes (devitrifies) to form the familiar snowflake shapes. The resultant glassy knots and crystals usually have a low amount of stress, but are nevertheless undesirable in glass articles.
While golf balls and glass windshields have teamed up for some notoriously, awful outcomes, metallic stones in glass containers are essentially their equal in terms of consequences. The metallic stones often form a sphere, presumably due to surface tension of the molten metal, but the dimples formed in this specimen evoked the cover of a golf ball. Compositional analysis revealed that it primarily was constituted by iron with a small amount of nickel. Thus, it was likely caused by the unplanned introduction of carbon steel or a cast iron impurity into the internal or external cullet. Although iron stones are not highly stressed, this particular stone was the origin and primary cause of breakage for this beverage container that failed during a retort operation under the influence of a thermal shock load.
Pagination
- Page 1
- Next page