Defect of the Month
Back to AGR's LibraryAlthough glass is valued for its purity and chemical inertness, certain conditions can foster interactions between a container and the product. Utilizing untreated bottles for certain alcoholic products with an approximately neutral pH might cause it to “snow” in July. Alcoholic beverages, such as vodka or gin, are generally not buffered against changes in pH. Over time, alkali leaching from the glass can cause the pH of the product to increase, which becomes more alkaline. If the pH rises high enough, the solution will attack the glass surface, resulting in siliceous glass flakes or particles in the product.
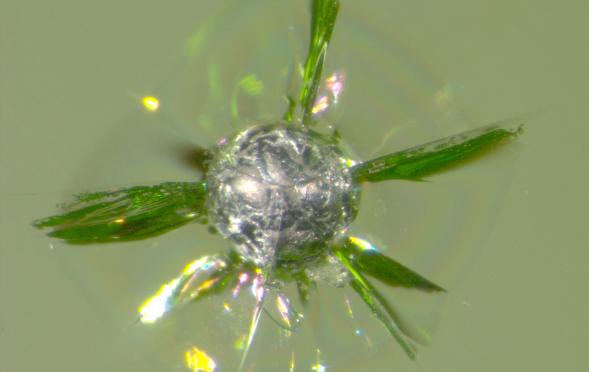
Like a dandelion blooming in your yard, this stone in glass is an unwelcome addition to a glass container. The smooth grey surface belies extremely high tensile stresses in the surrounding glass, created by the stone’s lower coefficient of thermal expansion. Silicon balls such as this stone are created when aluminum contamination in the cullet reacts with the glass melt, creating elemental silicon and aluminum oxide. When the aluminum oxide dissolves, only the silicon sphere remains. The high induced stresses sometimes cause spontaneous cracking, such as in this stone, or spontaneous fracture sometime during processing. For that reason, silicon balls are among the very worst of stones!
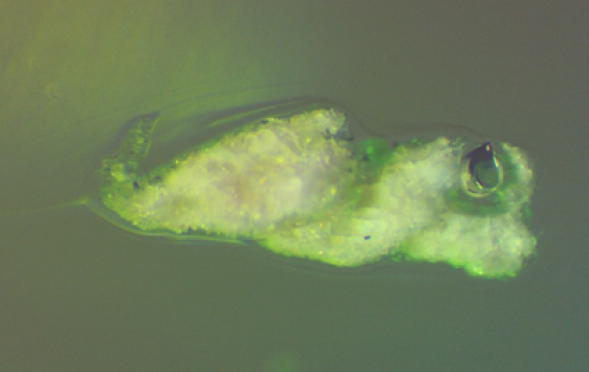
No, that isn’t a new yoga pose! With a little bit of whimsy and imagination, this photomicrograph of a stone in glass looks like a frog swimming through green water. The cracked surface texture and tight solution sac are clues as to its origin, since both traits are characteristic of aluminosilicate refractory material. When this stone was analyzed via SEM-EDX, the interior consisted of recrystallized alumina and nepheline – both are commonly created by the reaction of an alumina-rich refractory with the glass melt.
Perhaps nothing says “cozy” like an image of grandma in a rocking chair, knitting a blanket with a big basket of yarn at her feet. The bunches of crystals in this Scanning Electron Microscope image appear remarkably similar to jumbled skeins of yarn, but have a very different source. They were found inside a molybdenum blister created in the glass melt when boosting electrodes were exposed to air in the furnace. The crystals themselves are composed primarily of sodium molybdate with a smaller amount of calcium molybdate.
Pagination
- Previous page
- Page 2
- Next page